TALENT FOR THE REALITIES OF RESEARCH
TALENT FOR THE REALITIES OF RESEARCH
ON-DEMAND TALENT BUILT FOR THE SPEED OF CLINICAL TRIALS
WHY SPONSORS AND SITES CHOOSE RAPIDTRIALS
For more than two decades, RapidTrials has partnered with sponsors, CROs, and research sites to strengthen studies with dependable, well‑matched clinical research professionals. Our approach is grounded in firsthand operator-side experience, giving us real-world insight into how sites work and what it takes to run a study day-to-day.
FAST, HIGH‑QUALITY
HIRING
Access rigorously vetted clinical research staff who can step into active trials quickly. We match talent to protocol demands, site workflows, and therapeutic area expertise.
INFORMED BY REAL-
WORLD SITE OPERATIONS
Our team has lived the realities of clinical operations—from patient intake and scheduling to protocol navigation, data timelines, IRB submissions, and recruitment pressure. We understand what trial teams need because we’ve done the work.
FLEXIBLE STAFFING
MODELS
Direct‑hire, project‑based, interim leadership, remote/decentralized roles, embedded staffing, hybrid models, or support pods for multisite studies.
90% REPEAT
CLIENTS
Sponsors and sites return because our people deliver consistently, communicate clearly, integrate seamlessly, and maintain study momentum across timelines, geographies, and therapeutic areas.
CLINICAL TRIAL STAFFING SOLUTIONS
RapidTrials provides specialized, trial‑ready professionals across the clinical research ecosystem—from core site roles to centralized services to study leadership.
Interdisciplinary & Functional Teams
Cross‑functional specialists supporting patient management, scheduling, recruitment, retention, regulatory tracking, data flow, visit coordination, and study startup.
Traditional Research Site Roles
Centralized Support for Multi-site Studies
Executive & Leadership Recruitment
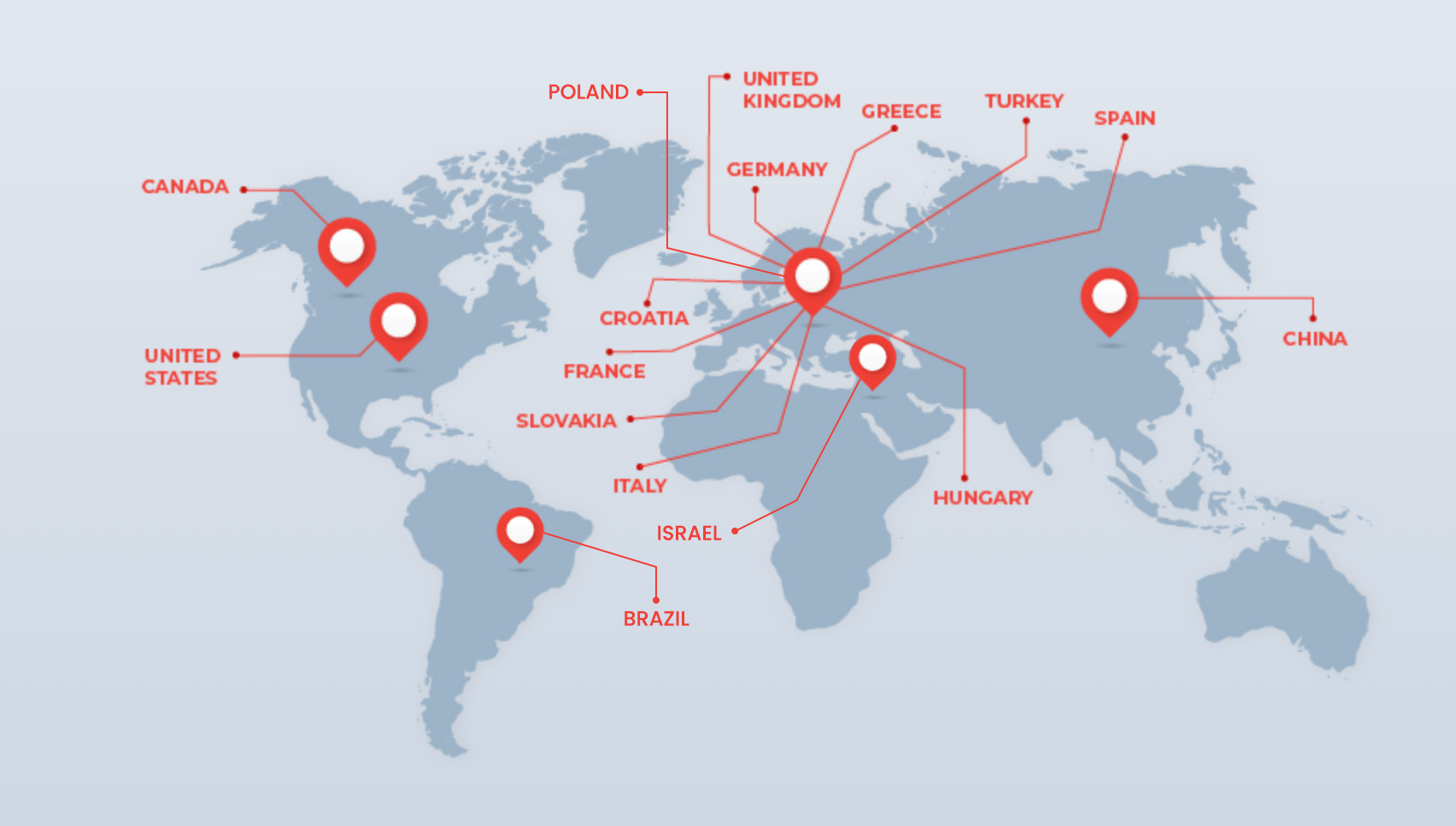
GLOBAL REACH, LOCAL UNDERSTANDING
RapidTrials supports staffing across North America, Europe, and additional global regions. Our vetted global network enables rapid deployment, regional familiarity, and consistent performance across diverse study locations.