In the rapidly evolving landscape of clinical research, Decentralized Clinical Trials (DCTs) are revolutionizing how medical innovations reach patients. By leveraging technology and flexible trial designs, DCTs make research more accessible, efficient, and patient-centric. At RapidTrials, we’re at the forefront of this transformation, empowering biopharmaceutical companies and research sites with tailored talent solutions to navigate the complexities of DCTs. In this blog, we explore the rise of decentralized clinical trials, their impact on the industry, and how RapidTrials is driving success in this space.
What Are Decentralized Clinical Trials?
Decentralized Clinical Trials use digital tools, remote monitoring, and virtual engagement to conduct research outside traditional clinical settings. By incorporating technologies like telemedicine, wearable devices, and mobile apps, DCTs allow patients to participate from their homes, reducing the need for frequent site visits. This approach enhances patient recruitment, improves retention, and accelerates trial timelines.
According to a 2024 report from Clinical Leader, DCTs have gained significant traction, with over 60% of clinical trial sponsors adopting decentralized or hybrid models to improve efficiency. The FDA’s 2024 Final Guidance on DCTs further solidified their importance, emphasizing patient safety and data integrity while encouraging innovative trial designs.
Why Decentralized Clinical Trials Are Trending in 2025
The shift toward DCTs is driven by several key factors reshaping clinical research:
- Patient-Centric Approach: DCTs prioritize convenience, enabling participants to engage in trials without disrupting their daily lives. This is especially critical for underserved populations or those in remote areas.
- Technological Advancements: Wearable devices, eConsent platforms, and AI-driven data analytics have made remote trial management seamless and reliable.
- Regulatory Support: The FDA’s 2024 guidance provides a clear framework for implementing DCTs, boosting confidence among sponsors and Contract Research Organizations (CROs).
- Talent Shortages: A chronic shortage of clinical site professionals, such as principal investigators and site coordinators, has pushed the industry toward flexible, decentralized models that require specialized talent. RapidTrials addresses this gap with expert talent management services.
As noted in a 2025 Fierce Biotech article, the global DCT market is projected to grow at a CAGR of 14.8% through 2030, driven by the need for faster, more inclusive trials. This trend underscores the urgency for sponsors and CROs to adapt to decentralized models to stay competitive.
How RapidTrials Powers Decentralized Clinical Trials
With over 25 years of experience in clinical operations, RapidTrials is a trusted partner for global biopharmaceutical companies and medical research centers. Our expertise in talent management and site enablement ensures that DCTs are executed with precision and efficiency. Here’s how we make it happen:
1. Building High-Functioning Teams
RapidTrials sources talent that aligns with your study’s goals and organizational culture. Whether you need remote coordinators, hybrid teams, or on-site professionals, we deliver candidates with the skills to excel in decentralized settings.
Ready to build your DCT team? Hire talent with RapidTrials today!
2. Evidence-Based Strategies
Our 25-year track record informs our approach to talent planning, workforce optimization, and compliance. We use data-driven insights to ensure your trial stays on track, even in complex decentralized environments.
3. Global Reach
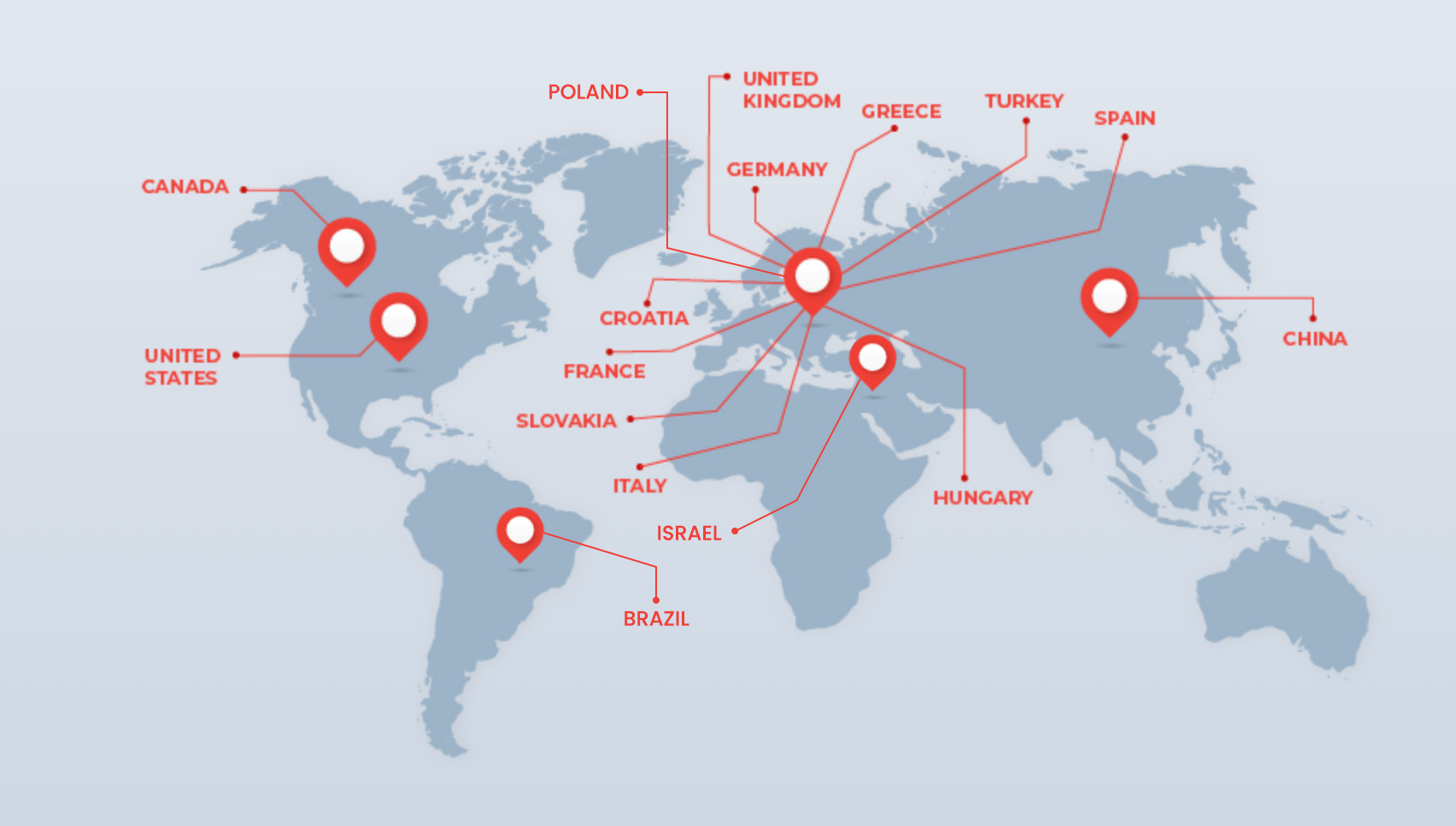
With a worldwide network, RapidTrials connects you with top talent across 13 countries, including the U.S., UK, and China. Our global presence supports seamless DCT implementation, no matter the location.
4. Flexibility and Agility
DCTs demand adaptable solutions. RapidTrials offers flexible staffing options, including direct hires, contractors, and project-based consultants, to meet the unique needs of decentralized trials.
Looking for a career in clinical research? Explore job opportunities with RapidTrials and join the future of DCTs!
5. Site Enablement Services
From workforce planning to payroll processing, our comprehensive site enablement solutions streamline DCT operations.
The Future of Clinical Research with DCTs
The rise of DCTs signals a new era in clinical research, one that prioritizes accessibility, efficiency, and innovation. However, the success of these trials hinges on having the right talent and strategies in place. RapidTrials is uniquely positioned to address these challenges, offering tailored solutions that empower sponsors, CROs, and sites to achieve their goals.
As the industry continues to embrace decentralized models, RapidTrials remains a leader in navigating this transformation. Our expertise, global reach, and evidence-based approach make us the go-to partner for DCT success.
Get Started with RapidTrials Today
Ready to transform your clinical trials with decentralized solutions? Partner with RapidTrials to access top-tier talent and site enablement services. Whether you’re a sponsor looking to hire talent or a professional seeking job opportunities, we’re here to help.