Clinical trial sponsors (Sponsors) and Contract Research Organizations (CROs) each play a critical role in ensuring that medical institutions conduct trials comply with complex staffing regulations. Regulatory oversight extends across professional licensing, employment classification, workplace safety, and insurance requirements. Ensuring compliance not only mitigates legal and financial risks but also enhances trial integrity and efficiency. This white paper explores key regulatory considerations impacting clinical trial staff and outlines how Sponsors and CROs can utilize specialized clinical research staffing firms to assist sites in achieving compliance and optimal outcomes.
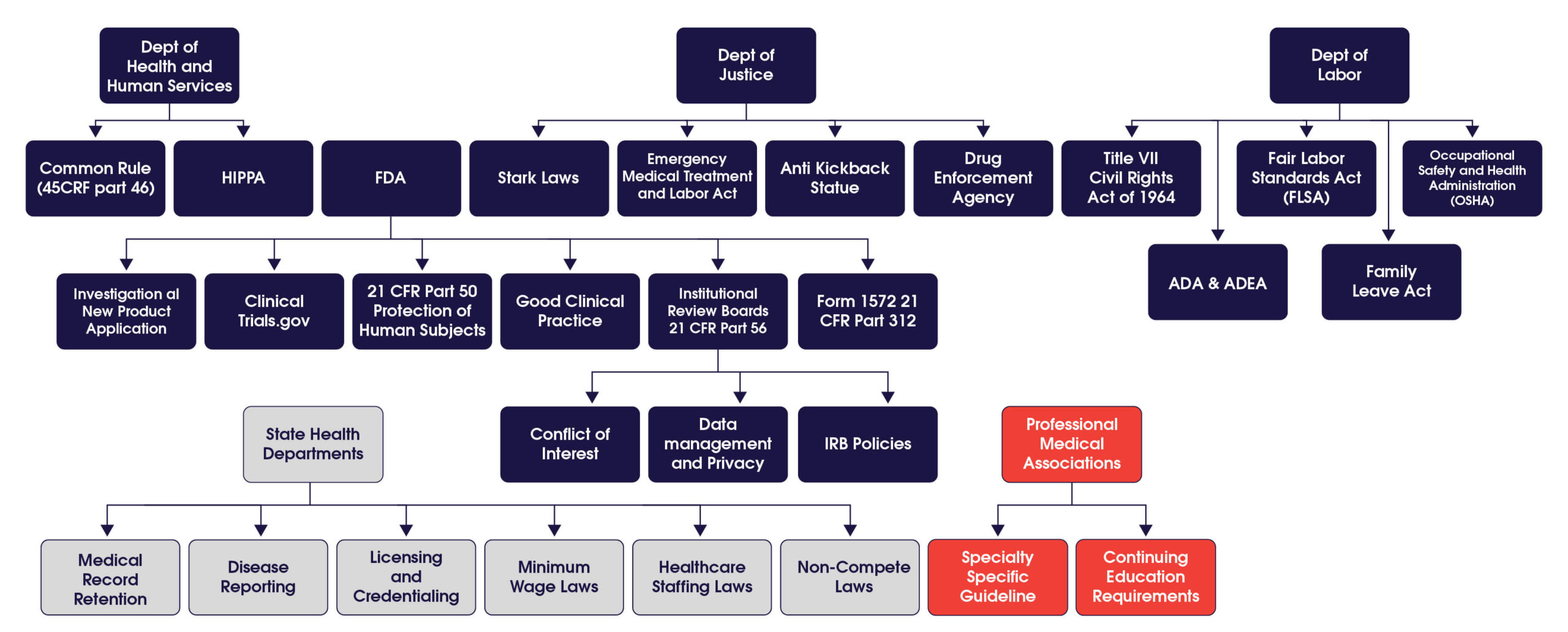
The illustration1. Key US Federal, State, and Professional Organizations That Shape the Work of Clinical Researchers
Providing trial sites with state-by-state regulatory requirements to ensure all healthcare professionals meet necessary licensing mandates.
Sponsors and CROs can utilize systems to verify and track licenses and certifications of clinical personnel across multiple trial sites in the United States and abroad.
Offering funding and access to training for Clinical Research Coordinators (CRCs) and Clinical Research Associates (CRAs) to maintain Good Clinical Practice (GCP) compliance.
Helping sites navigate IRB approval processes to ensure proper compliance with ethical and regulatory requirements.
Establishing clear guidelines and disclosure requirements to prevent conflicts of interest under the Physician Payments Sunshine Act.
Ensuring personnel handling patient data are trained in HIPAA regulations to protect confidentiality and avoid data breaches.
Providing training modules to ensure staff comply with FDA and HHS regulations (21 CFR Part 50, 45 CFR Part 46) in obtaining informed consent from trial participants.
Assisting sites in correctly classifying research personnel to prevent misclassification fines under the Fair Labor Standards Act (FLSA) and Department of Labor (DOL) regulations.
Helping sites implement compliant payroll systems to ensure proper compensation and benefits for employees.
Providing guidance on overtime requirements and Family and Medical Leave Act (FMLA) protections to avoid labor law violations.
Supporting sites with workplace safety training and audits to ensure adherence to OSHA Bloodborne Pathogens and Hazard Communication (HazCom) standards.
Ensuring trial sites have access to necessary PPE and safety training for staff working with biological specimens or hazardous materials.
Helping trial sites obtain Errors & Omissions Insurance to cover trial design risks and regulatory compliance gaps.
Assisting sites in securing malpractice insurance for physicians and healthcare professionals involved in trials.
Providing recommendations for insurance providers that cover trial-related third-party claims, including property damage and bodily injury.
Ensuring trial sites maintain required workers’ compensation coverage for employees at risk of job-related injuries.
Creating centralized hiring initiatives or partnerships with staffing agencies specializing in clinical research.
Establishing a dedicated resource to help trial sites identify, recruit, and onboard qualified clinical research personnel.
Providing tools to help sites anticipate and mitigate staffing shortages.
Implementing platforms that monitor compliance metrics across multiple trial sites.
Supporting site personnel in obtaining necessary certifications and ongoing regulatory training.
Assisting sites with risk assessments to ensure legal and ethical compliance.
Regularly evaluate trial sites to ensure compliance with licensing, labor laws, and insurance mandates.
Create dedicated teams that work closely with trial sites to monitor regulatory requirements and mitigate risks. Side benefit of close communication aids in discovering issues early in the process and bring all resources to focus on eliminating any blockages to accomplishing goals
Develop and deploy training modules that ensure all trial personnel understand regulatory obligations.
Utilize technology to track personnel licensing and certification across multiple sites.
Ensure trial sites have access to additional staff or means to quickly onboard any resources that may be in short supply and regulatory guidance during periods of personnel shortages.
Enhance Insurance and Liability Oversight
Assist trial sites in maintaining the appropriate level of insurance coverage for clinical trial risks.
1. U.S. Food and Drug Administration (FDA) Regulations – Clinical Trials and Human Subject Protection [https://www.fda.gov/science-research/science-and-research-special-topics/clinical-trials-and-human-subject-protection]
2. Department of Health and Human Services (HHS) – Common Rule Regulations [https://www.hhs.gov/ohrp/regulations-and-policy/regulations/common-rule/index.html]
3. Occupational Safety and Health Administration (OSHA) – Healthcare Industry Safety Standards [https://www.osha.gov/healthcare]
4. Fair Labor Standards Act (FLSA) – U.S. Department of Labor [https://www.dol.gov/agencies/whd/flsa]
5. Association of Clinical Research Professionals (ACRP) – Certification Standards [https://www.acrpnet.org/certifications]
6. Society of Clinical Research Associates (SoCRA) – Certification Program [https://www.socra.org/certification]
7. National Institutes of Health (NIH) – Good Clinical Practice Training [https://grants.nih.gov/policy/humansubjects/gcp.htm]
Clinical trial sponsors (Sponsors) and Contract Research Organizations (CROs) each play a critical role in ensuring that medical institutions conduct trials comply with complex staffing regulations. Regulatory oversight extends across professional licensing, employment classification, workplace safety, and insurance requirements. Ensuring compliance not only mitigates legal and financial risks but also enhances trial integrity and efficiency. This white paper explores key regulatory considerations impacting clinical trial staff and outlines how Sponsors and CROs can utilize specialized clinical research staffing firms to assist sites in achieving compliance and optimal outcomes.
The illustration1. Key US Federal, State, and Professional Organizations That Shape the Work of Clinical Researchers

Providing trial sites with state-by-state regulatory requirements to ensure all healthcare professionals meet necessary licensing mandates.
Sponsors and CROs can utilize systems to verify and track licenses and certifications of clinical personnel across multiple trial sites in the United States and abroad.
Offering funding and access to training for Clinical Research Coordinators (CRCs) and Clinical Research Associates (CRAs) to maintain Good Clinical Practice (GCP) compliance.
Helping sites navigate IRB approval processes to ensure proper compliance with ethical and regulatory requirements.
Establishing clear guidelines and disclosure requirements to prevent conflicts of interest under the Physician Payments Sunshine Act.
Ensuring personnel handling patient data are trained in HIPAA regulations to protect confidentiality and avoid data breaches.
Providing training modules to ensure staff comply with FDA and HHS regulations (21 CFR Part 50, 45 CFR Part 46) in obtaining informed consent from trial participants.
Assisting sites in correctly classifying research personnel to prevent misclassification fines under the Fair Labor Standards Act (FLSA) and Department of Labor (DOL) regulations.
Helping sites implement compliant payroll systems to ensure proper compensation and benefits for employees.
Providing guidance on overtime requirements and Family and Medical Leave Act (FMLA) protections to avoid labor law violations.
Supporting sites with workplace safety training and audits to ensure adherence to OSHA Bloodborne Pathogens and Hazard Communication (HazCom) standards.
Ensuring trial sites have access to necessary PPE and safety training for staff working with biological specimens or hazardous materials.
Helping trial sites obtain Errors & Omissions Insurance to cover trial design risks and regulatory compliance gaps.
Assisting sites in securing malpractice insurance for physicians and healthcare professionals involved in trials.
Providing recommendations for insurance providers that cover trial-related third-party claims, including property damage and bodily injury.
Ensuring trial sites maintain required workers’ compensation coverage for employees at risk of job-related injuries.
Creating centralized hiring initiatives or partnerships with staffing agencies specializing in clinical research.
Establishing a dedicated resource to help trial sites identify, recruit, and onboard qualified clinical research personnel.
Providing tools to help sites anticipate and mitigate staffing shortages.
Implementing platforms that monitor compliance metrics across multiple trial sites.
Supporting site personnel in obtaining necessary certifications and ongoing regulatory training.
Assisting sites with risk assessments to ensure legal and ethical compliance.
Regularly evaluate trial sites to ensure compliance with licensing, labor laws, and insurance mandates.
Create dedicated teams that work closely with trial sites to monitor regulatory requirements and mitigate risks. Side benefit of close communication aids in discovering issues early in the process and bring all resources to focus on eliminating any blockages to accomplishing goals
Develop and deploy training modules that ensure all trial personnel understand regulatory obligations.
Utilize technology to track personnel licensing and certification across multiple sites.
Ensure trial sites have access to additional staff or means to quickly onboard any resources that may be in short supply and regulatory guidance during periods of personnel shortages.
Enhance Insurance and Liability Oversight
Assist trial sites in maintaining the appropriate level of insurance coverage for clinical trial risks.
1. U.S. Food and Drug Administration (FDA) Regulations – Clinical Trials and Human Subject Protection [https://www.fda.gov/science-research/science-and-research-special-topics/clinical-trials-and-human-subject-protection]
2. Department of Health and Human Services (HHS) – Common Rule Regulations [https://www.hhs.gov/ohrp/regulations-and-policy/regulations/common-rule/index.html]
3. Occupational Safety and Health Administration (OSHA) – Healthcare Industry Safety Standards [https://www.osha.gov/healthcare]
4. Fair Labor Standards Act (FLSA) – U.S. Department of Labor [https://www.dol.gov/agencies/whd/flsa]
5. Association of Clinical Research Professionals (ACRP) – Certification Standards [https://www.acrpnet.org/certifications]
6. Society of Clinical Research Associates (SoCRA) – Certification Program [https://www.socra.org/certification]
7. National Institutes of Health (NIH) – Good Clinical Practice Training [https://grants.nih.gov/policy/humansubjects/gcp.htm]

RapidTrials is a global leader in clinical trial talent management, with over 25 years of experience in accelerating clinical research through strategic workforce solutions. We specialize in designing, hiring, and supporting high-performing study teams for CROs, pharmaceutical companies, and life science organizations. Our data-driven approach and extensive industry expertise ensure that your clinical trials are staffed with the right professionals, enhancing efficiency and trial outcomes.